
Prof. Lixin Zhang(张立新)
- Director, State Key Laboratory of Bioreactor Engineering,
- Deputy Dean, School of Biotechnology,
- East China University of Science and Technology,
- Room 18-201, 130 Meilong Rd, Shanghai, 200237, China
- Tel/Fax: CN +86-021-64252575 (Office) +86-021-64253020 (lab)
- E-mail: lxzhang@ecust.edu.cn
- Editor-in-Chief, Synthetic and Systems Biotechnology
- Associate Editor-in-Chief, Applied Microbiology and Biotechnology
- Associate Editor-in-Chief, Frontiers in Bioengineering and Biotechnology
- First Ambassador of Federation of European Microbiological Societies in China
- Co-founder & Former-President, International Chemical Biology Society
- Former-Ambassador of American Society for Microbiology in China
- ORCID: 0000-0003-0894-9520
Research Highligts
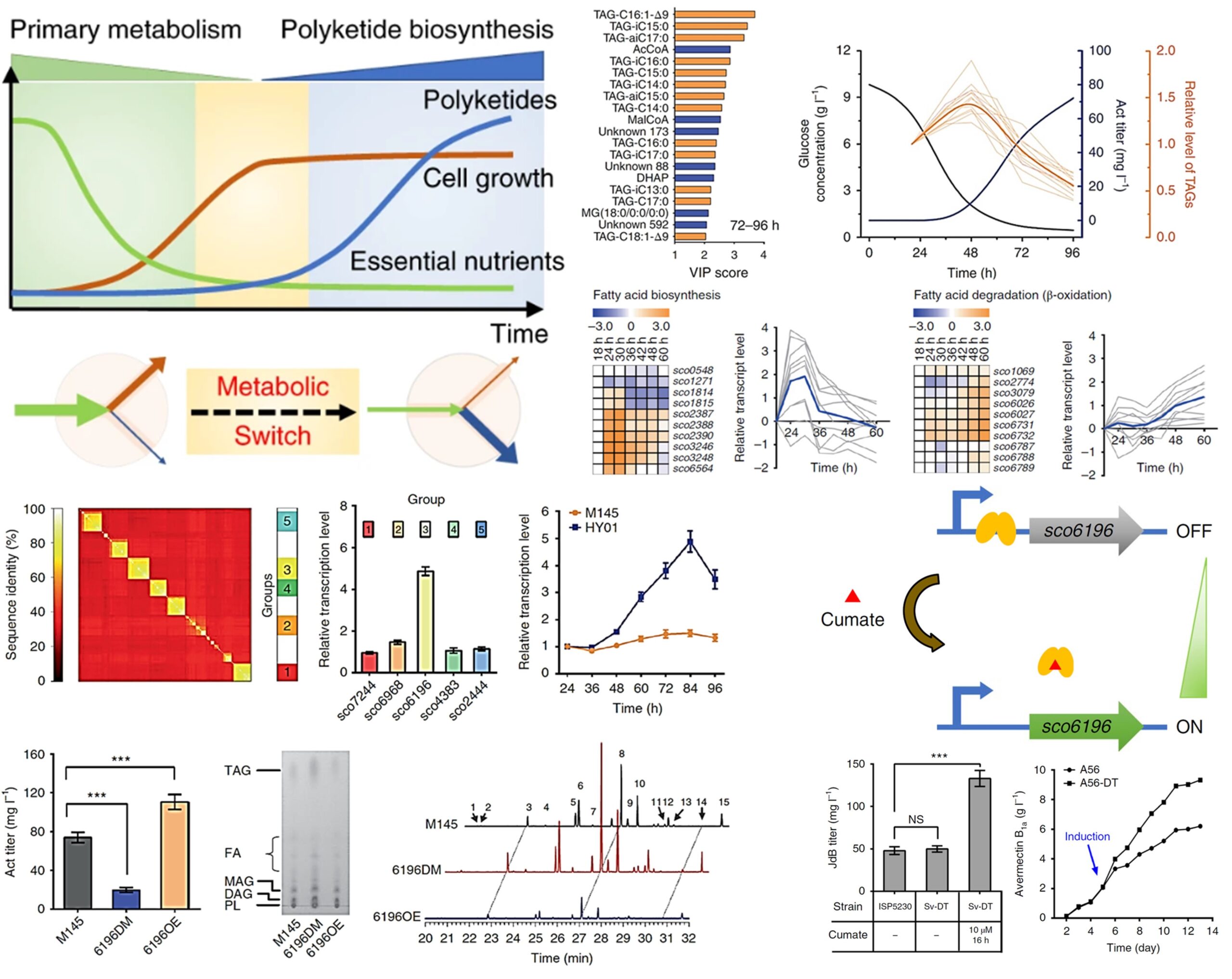
Polyketides are mainly produced as secondary metabolites during the stationary phase of growth of Streptomyces species. The source of intracellular metabolites that are funneled into polyketide biosynthesis has proven elusive. We applied multi-omics to reveal that intracellular triacylglycerols (TAGs), which accumulates in primary metabolism, are degraded during stationary phase. This process could channel carbon flux from intracellular TAGs and extracellular substrates into polyketide biosynthesis. We devised a strategy named ‘dynamic degradation of TAG’ (ddTAG) to mobilize the TAG pool and increase polyketide biosynthesis. Our strategy could improve polyketide titers for pharmaceutical production. (Nat Biotechnol, 2020, 38:76-83).
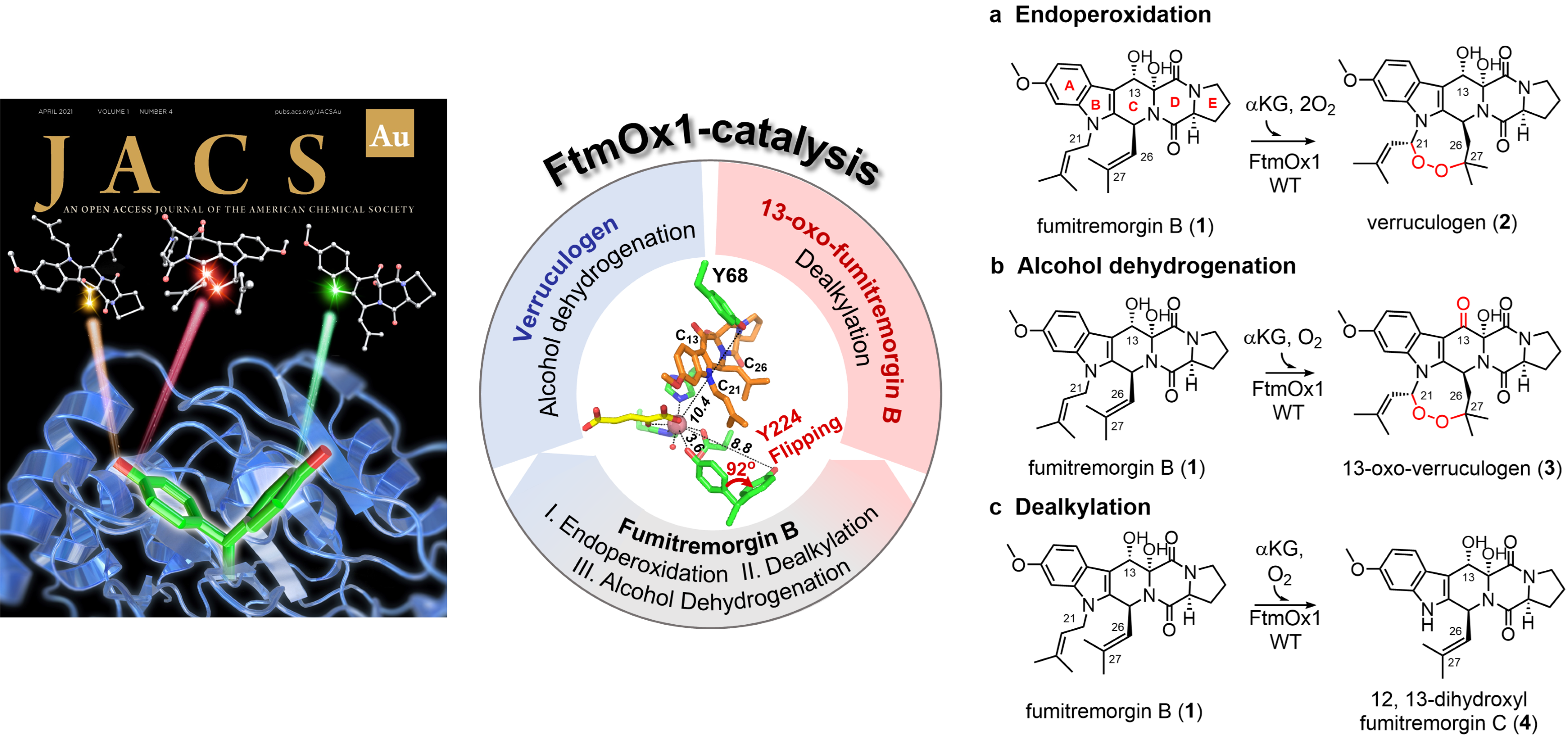
FtmOx1 is a non-heme iron (NHFe) endoperoxidase, catalyzing three disparate reactions, endoperoxidation, alcohol de-hydrogenation, and dealkylation, under in vitro conditions; the diversity complicates its mechanistic studies. Recently, we used substrate analogs to simplify the FtmOx1-catalyzed reaction from a very complicated system with three reactions to predominantly either the dealkylation or an alcohol dehydrogenation reaction. These simplified systems served as excellent models for FtmOx1 mechanistic characterizations using kinetic/spectroscopic/structural methods, and the results are more consistent with the COX-like model. (JACS Au 2022 2 (7), 1686-1698).
Research Interest
Translate Microbial Natural Products into drugs for life-threatening diseases. Our research is focused on:
1. Diversifying marine microbial natural product strains and extract libraries, while decreasing genetic and chemical redundancy.
2. Screening for synergistic medicines in a high throughput manner.
3. Increasing the production of drugable secondary metabolites from microbial producers by genetic engineering.
Biography
Prof. Lixin Zhang served as the Director of National Key Laboratory of Bioreactor Engineering at East China University of Science and Technology. His research focused on: Taxonomy guided diversification of a marine microbial natural product library; screening for synergistic medicines in a high throughput manner; increasing the production of drugable secondary metabolites from microbial producers by synthetic biology. His Avermectin project won a National Award for “Excellence to improve science and technologies” in 2017. He was a Chief Scientist of a “973 Program” and an Awardee of the National Science Fund for Distinguished Young Scholars, China. He worked in 3 pharmaceutical companies in USA and published seven books, 288 papers and holds 28 Chinese patents and 16 PCT patents. He served as the President of the International Chemical Biology Society (ICBS) during 2016-8. He has been appointed as an Editor-in-Chief for “Synthetic and Systems Biotechnology”, Associate Editor-in-Chief for “Applied Microbiology and Biotechnology”, etc. He also served as an Executive Board Member of International Committee on the Biology of Actinomycetes (ISBA) and the GIM (Genetics of Industrial Microorganisms). He is also on the SYNBIOCHEM External Advisory Board for the Manchester Institute of Biotechnology.